2 Methyl 2 Pentene Hbr
Main Organic Chemistry Reaction Guide
Addition of HBr to Alkenes
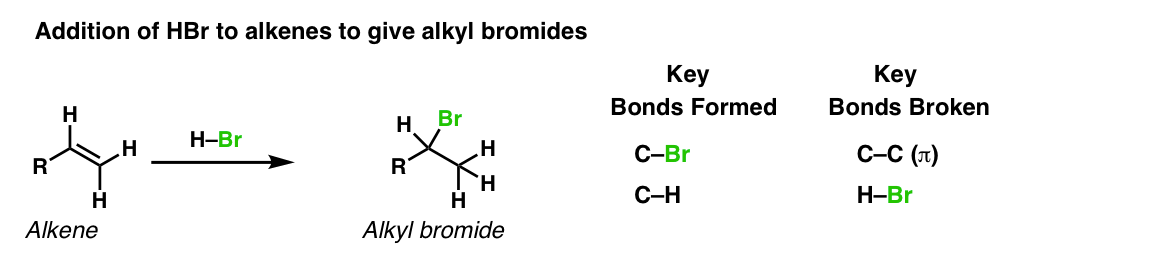
Description: Treatment of alkenes with hydrobromic acid will result in the formation of alkyl bromides.
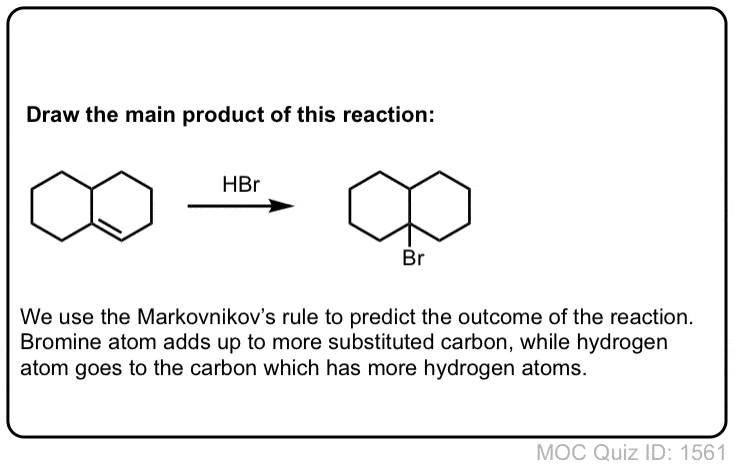
Notes: This is an addition reaction. Note that the bromine always ends upward at the more substituted carbon of the alkene (Markovnikoff-selectivity)
Examples:
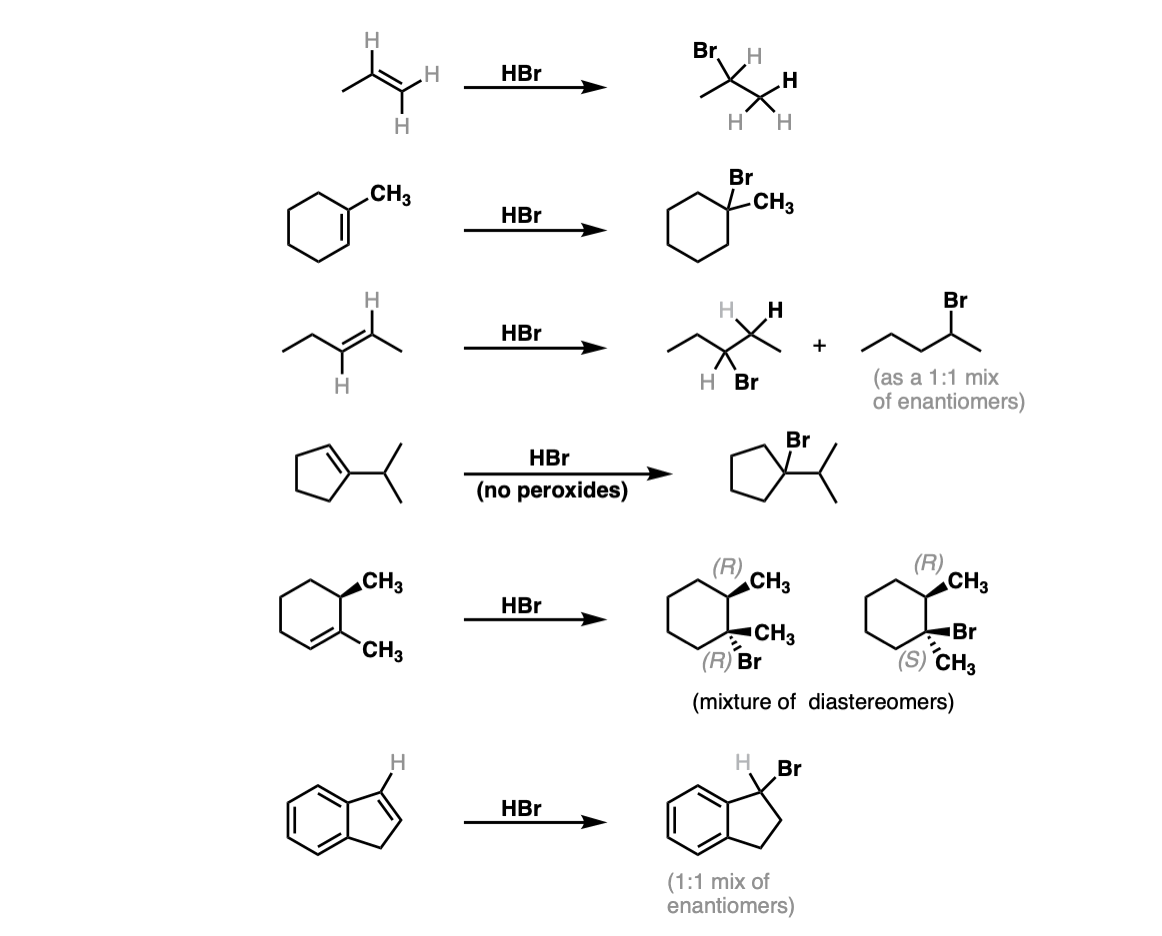
Notes: The first two examples show a simple improver of HBr to requite the Markovnikov product. In the third instance Markovnikov'south rule gives no articulate preference, so a mixture is obtained. The fifth case gives a mixture of diastereomers, since addition of HBr across the alkene will not affect the initial (R) stereochemistry. The final (sixth) examplewill requite a dominant product, despite identical substitution, because germination of the resonance-stabilized carbocation will exist favored!
When a secondary carbocation is formed adjacent to a third or quaternary carbon, rearrangements are possible. See these pages for more examples:
- Addition with ane,2- hydride shift
- Add-on with 1,2- alkyl shift
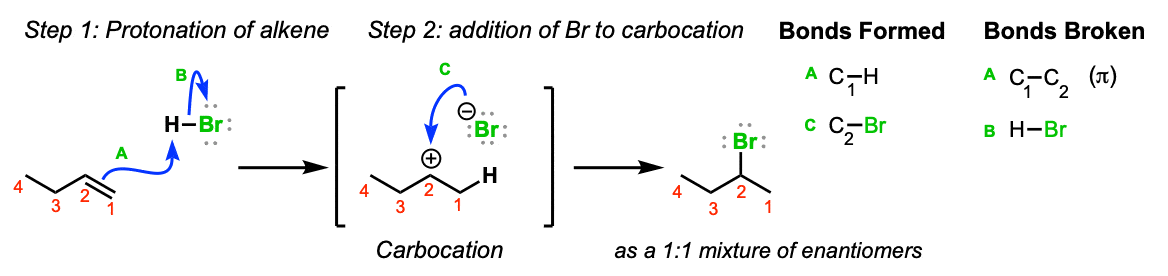
Machinery: Electrons from the C1-C2 π bond assault the hydrogen of HBr, expelling the bromide anion and leading to the formation of a carbocation (Step one, arrows A and B). Annotation hither that the carbocation preferentially forms on C2 (secondary) and not C1 (primary) since secondary carbocations are more stable. The bromide anion and then attacks the carbocation, leading to formation of the alkyl bromide (Stride 2, arrow C)
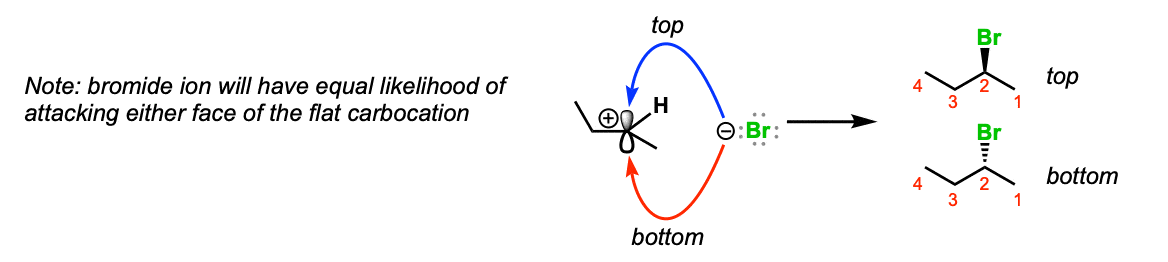
Notes: Since the carbocation is planar (apartment) at that place is no preferred management of assail of the bromide ion. If information technology's possible to course a stereocenter, a mixture of stereoisomers volition be obtained.
Rearrangements: When secondary (or chief) carbocations are formed adjacent to a more substituted carbon, side by side hydrogen atoms or alkyl groups can shift, resulting in formation of a more stable carbocation.
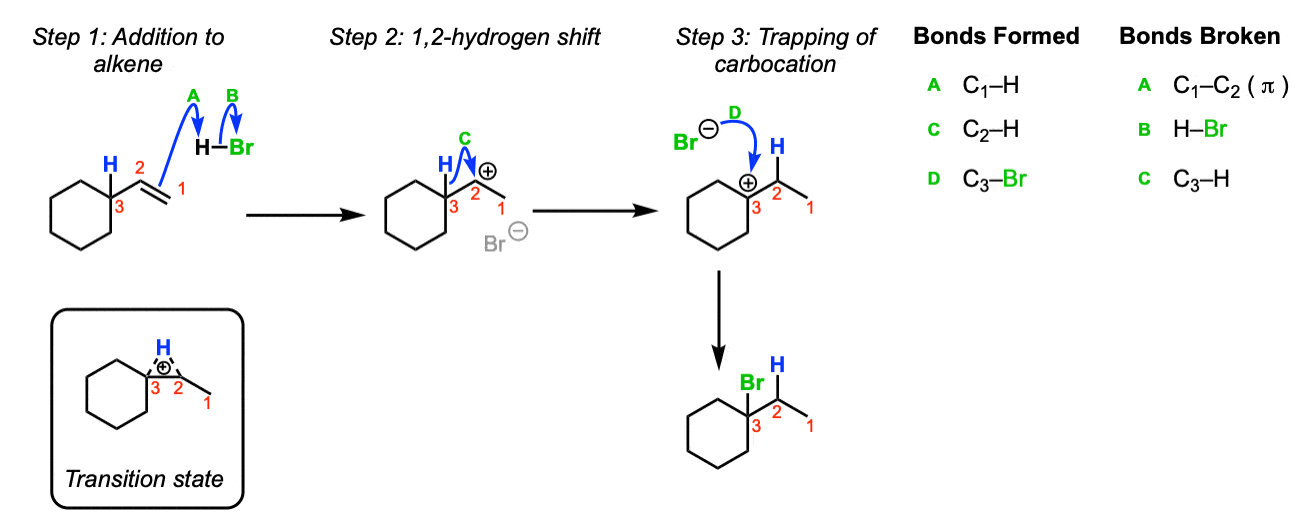
The offset step of the reaction below is formation of a carbocation. Here, the formation of a carbocation via attack of the alkene upon H-Br is shown. (Stride ane, arrows A and B). Since we take a secondary carbocation adjacent to a tertiary carbon, shift of a hydrogen to the secondary carbocation will consequence in a (more than stable) tertiary carbocation (Pace 2, arrow C). The tertiary carbocation is then trapped, in this case, by Br(–) (Step 3, arrows D). The rearrangement pace goes through a transition state such every bit the one pictured.
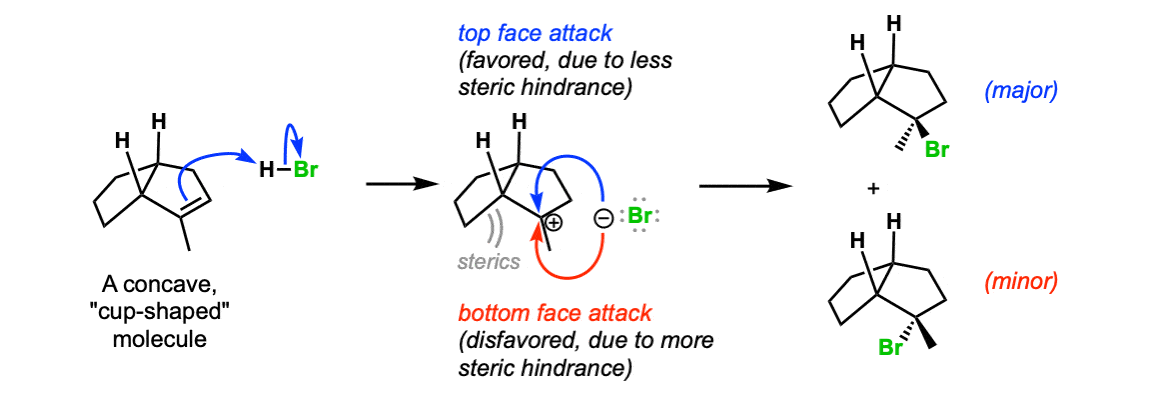
Additional example (advanced) : If the molecule contains next stereocenters, the two directions of attack on the carbocation will no longer be of equal free energy and a mixture of diastereomers volition be obtained. In this instance, attack of the bromide ion on the less hindered "superlative face" of the carbocation is favored, and a mixture of products (diastereomers) will be obtained.
Rearrangements tin can also occur. See Additions To Alkenes Accompanied Past 1,2-Hydride Shifts
Quiz Yourself!
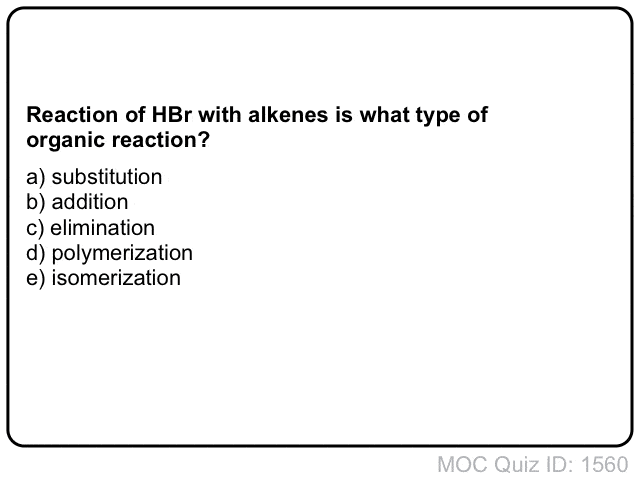 Click to Flip
Click to Flip
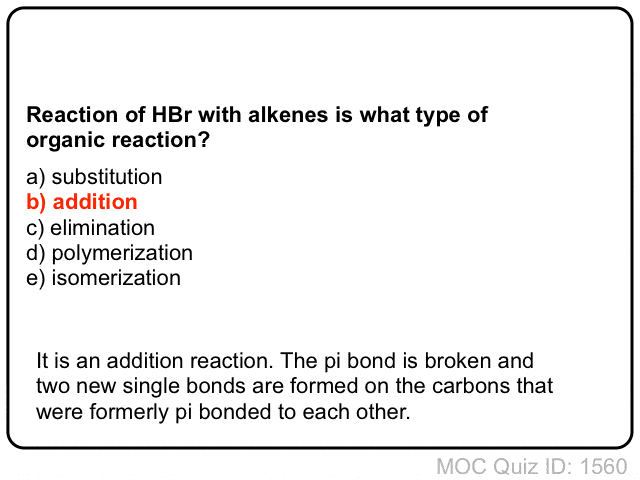
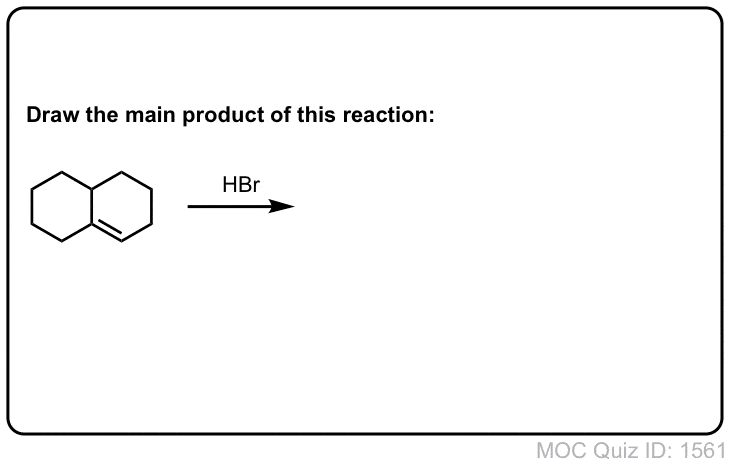 Click to Flip
Click to Flip
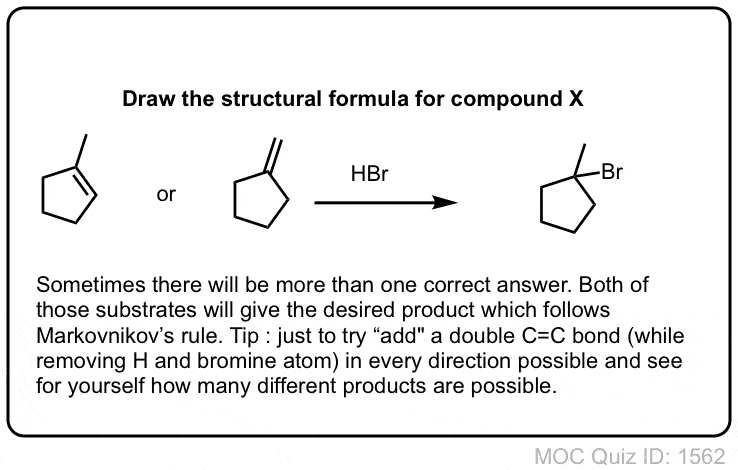
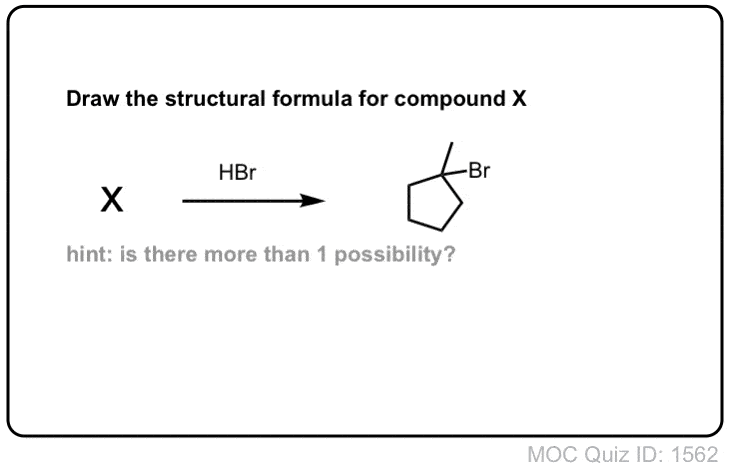 Click to Flip
Click to Flip
(Advanced) References And Further Reading:
- Early on case
The Stereochemistry of the Addition of Hydrogen Bromide to ane,2-Dimethylcyclohexene
George South. Hammond and Thomas D. Nevitt
Journal of the American Chemical Society 1954 76 (16), 4121-4123
DOI: x.1021/ja01645a020
Early on paper from the l'south by Prof. George Hammond (of Hammond'southward Postulate) on the machinery of HBr addition to 1,2-dimethylcyclohexane. He prefers a concerted pathway, although that might due to the conditions he employs – in pentane, a very nonpolar solvent, polar intermediates are disfavored. - Mechanistic studies
Hydrochlorination of cyclohexene in acetic acrid. Kinetic and product studies
Robert C. Fahey, Michael Westward. Monahan, and C. Allen McPherson
Journal of the American Chemical Lodge 1970 92 (nine), 2810-2815
DOI: 10.1021/ja00712a034
Detailed kinetic studies of the improver of HCl to cyclohexene in acetic acid, discussing a possible 3rd-society machinery (rate = k[cyclohexene][HX]2). - Experimental Procedure
SPIROANNELATION OF ENOL SILANES:2-OXO-5-METHOXYSPlRO[5.iv]DECANE
Lee, T. V.; Porter, J. R. Org. Synth. 1995, 72, 189
DOI: ten.15227/orgsyn.072.0189
The offset reaction in the higher up process involves two steps – addition of HBr across the double bail and converting the aldehyde to a dimethyl acetal.
Real-Life Examples:
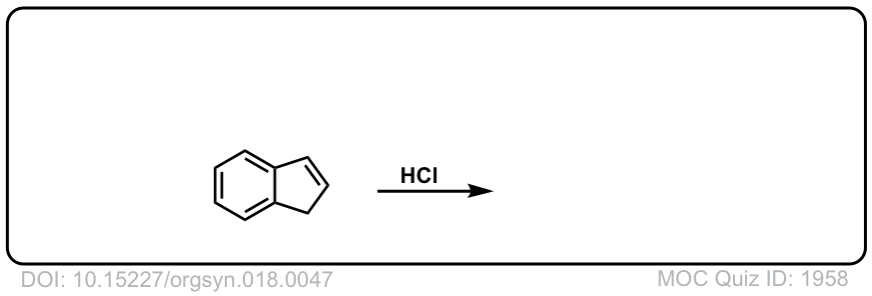
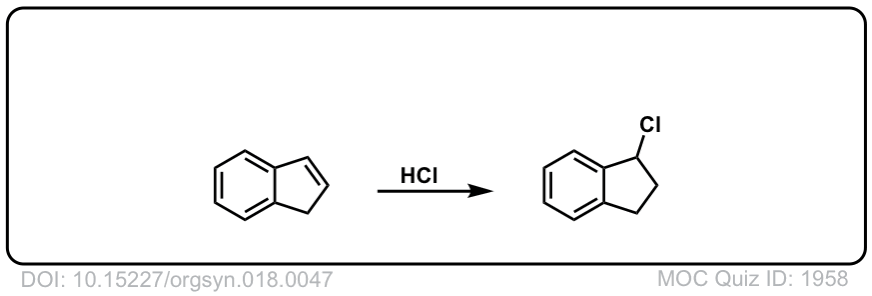
Org. Synth. 1938, 18, 47
DOI Link: 10.15227/orgsyn.018.0047
 Click to Flip
Click to Flip
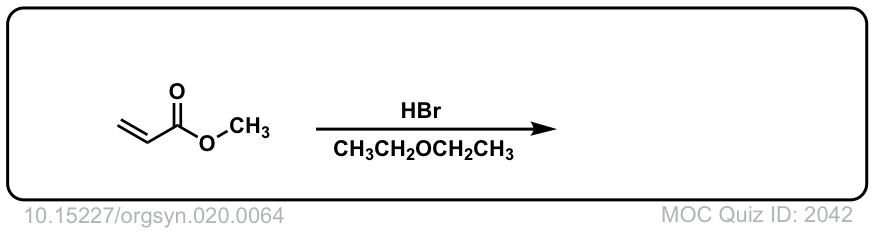
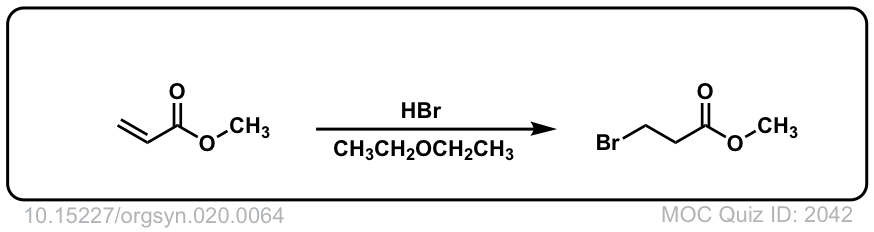
Org. Synth. 1940, 20, 64
DOI Link: 10.15227/orgsyn.020.0064
 Click to Flip
Click to Flip
2 Methyl 2 Pentene Hbr,
Source: https://www.masterorganicchemistry.com/reaction-guide/addition-of-hbr-to-alkenes/
Posted by: barnetthiscon.blogspot.com


0 Response to "2 Methyl 2 Pentene Hbr"
Post a Comment